[ Breastimplant ] Motiva, Motiva ergonomix
Motiva ergonomix
"
"Motiva (Ergonomix)" was approved by the US Food and Drug Administration (FDA) in 2018. Its safety and extremely low rate of capsular contracture have been well received in clinical practice and in the marketplace. Many mammoplasty patients have benefited."

Dr. Kikap Kim, a board-certified plastic surgeon specializing in mammoplasty for nearly 20 years and the Medical Director of the U&U Clinic, frequently represents in academic exchanges related to Motiva (Ergonomix).
In 2021, he was honored with the certification “Motiva Prestige New Leader” from the Motiva manufacturer.

"
Motiva Ergonomix Designed to move naturally with your body. We applied ergonomics principles to our design, challenging ourselves to come up with an implant that behaves as naturally as possible.
A breast implant designed to gently adapt and follow your body’s movements.
The MOTIVA breast implant is a nanotextured breast implant that has been proven safe with a capsular contracture rate of less than 1% since its introduction in 2010.

Benefits of Motiva ergonomix
Realistic feel
Motiva features an ultra-cohesive 4D flow gel with high elasticity and fluidity. This unique composition allows the implants to have a disc shape when lying down and a natural teardrop shape when standing, giving a natural and realistic look and feel. The gel moves naturally with the body for a lifelike effect.

Extremely low adverse events
Since its launch in 2010, Motiva has demonstrated a global capsular contracture rate of less than 1%, demonstrating its safety in the marketplace. The outer membrane material has been extensively developed and optimised into the SilkSurface™ nanosilk outer membrane. This surface covers the nano-sized fibres and promotes the formation of a thin, healthy capsule without the need for massage. The patented 3D Reverse Outer Membrane manufacturing technology, using only silicone material with no added foreign substances, significantly reduces the risk of allergies, capsular contracture and implant rupture.

High elasticity
Motiva’s design features a highly elastic outer membrane, which reduces the risk of rupture due to compression. In addition, the implants themselves have greater extensibility, allowing for smaller incisions during implantation and significantly reducing post-operative recovery time. Based on clinical experience, Motiva can reduce the incision size by approximately 1 centimetre compared to textured or teardrop gel implants.
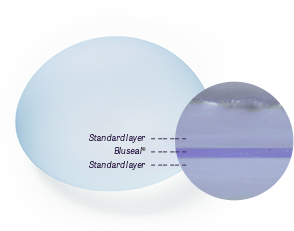
Also, due to Motiva’s detailed and sophisticated implant shell manufacturing technology, the ergonomic Smooth Silk™ shell has a high level of safety.